On February 17, Molnupiravir STELLA 400 was one of three official COVID-19 treatment drugs licensed for conditional circulation in Vietnam by the Ministry of Health and GONSA was pleased to be the National Distributor of the product.

Molnupiravir was indicated for the treatment of mild to moderate COVID-19 disease in adults with a positive diagnostic test for SARS-CoV-2 and with at least one risk factor for severe disease progression.
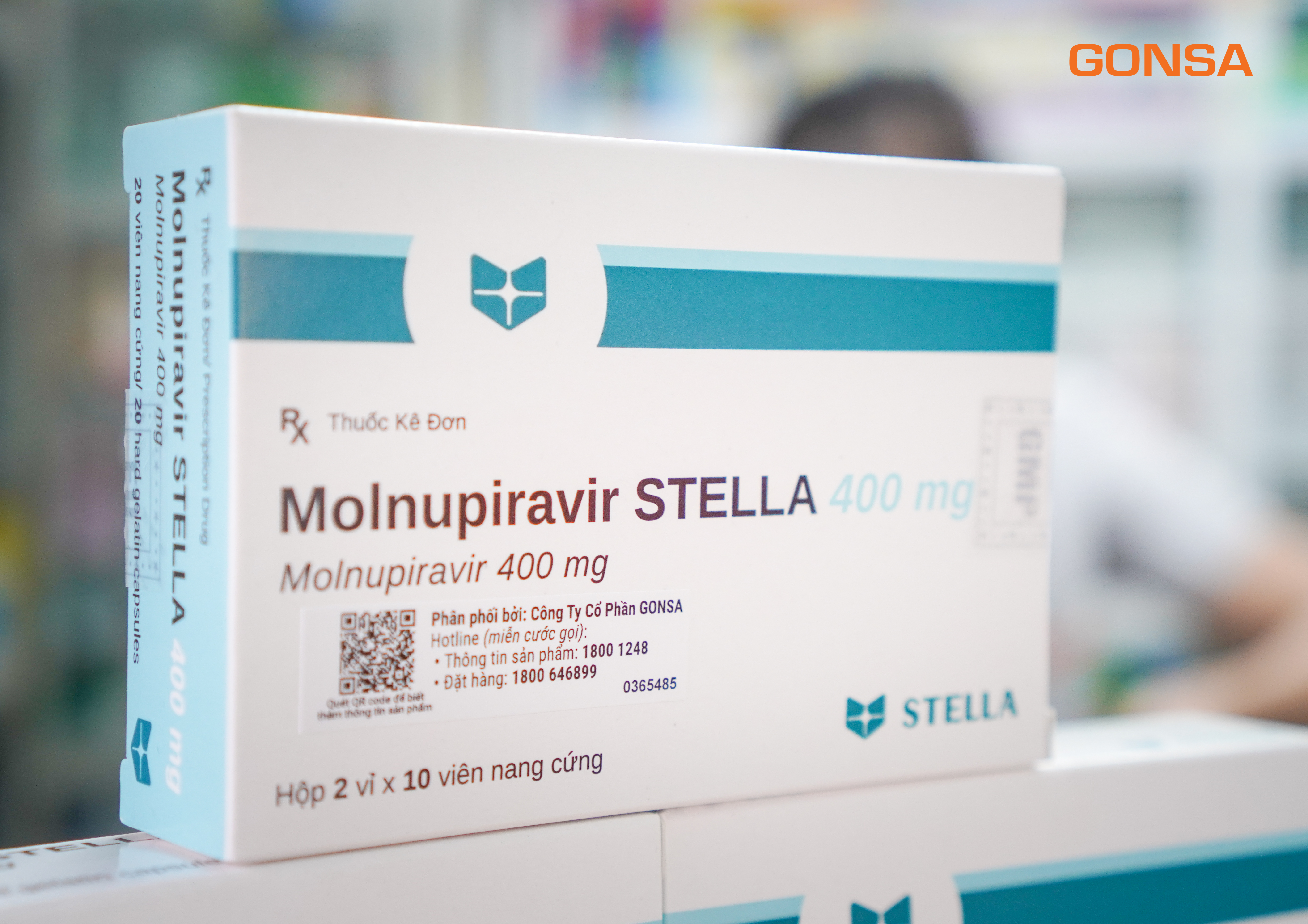
Molnupiravir drug box with QR code GONSA distributed in the box cover
Before licensed, Molnupiravir was tested in parallel clinical trials in the US, India and clinical trials at the treatment facilities of Thong Nhat Hospital, Faculty of Public Health, University of Medicine and Pharmacy Hospital of Ho Chi Minh City. The trial program has been conducted with over 80.000 patients in 51 provinces and cities across the country.

Molnupiravir is widely distributed by GONSA at drugstores nationwide
As a National Distributor, GONSA is and will always strive to make the drug supply the fastest and most widespread in the country and always ensure absolute compliance with the regulations of the Ministry of Health for with this particular product.
GONSA - SPECIALIZING IN PHARMACEUTICAL SERVICES

